Salinity, a critical measurement in oceanography, quantifies the concentration of salt dissolved in seawater and is typically expressed as “parts per thousand” (ppt). This metric is essential for understanding and characterizing the chemical composition of natural water bodies and its influence on various biological processes.
The classification of water as “brackish” sets a threshold at which salinity should not exceed 24.7%. When the quantity of dissolved salt surpasses 24.7 grams per 1,000 grams (or one kilogram) of water…
This threshold is used to distinguish brackish water from seawater, with brackish water having a lower salt content than the ocean. Salinity levels are vital for gauging the health of aquatic ecosystems, determining the distribution of marine species, and predicting how changes in salinity can impact marine life and the physical properties of the water. Researchers and environmental scientists closely monitor salinity levels in various water bodies to gain insights into ecological patterns and the overall health of aquatic environments.
Defination of ocean salinity
Salinity, when it comes to rivers, lakes, and oceans, is a concept that sounds straightforward but proves technically demanding to precisely define and measure. In essence, salinity represents the amount of dissolved salt within the water, with these salts being compounds such as sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate that break down into ions when they dissolve. Sometimes, the concentration of dissolved chloride ions is specifically termed as “chlorinity.”
To operationalize this concept, dissolved matter is defined as substances capable of passing through a highly fine filter, historically one with a pore size of 0.45 μm, but more commonly 0.2 μm nowadays. Salinity can be quantified in the form of a mass fraction, indicating the mass of dissolved substances within a unit mass of the solution.
Factors affecting ocean salinity
Evaporation’s Impact on Surface Salinity
When water in the ocean’s surface layer undergoes evaporation, fresh water is removed, leaving behind the dissolved salts. This process increases the salinity of the remaining water.
Precipitation’s Role in Surface Salinity
Conversely, precipitation introduces fresh water into the ocean. Rainfall dilutes the salinity of the surface layer, as it adds relatively salt-free water to the mix.
Coastal Freshwater Inflow
In coastal areas, the flow of freshwater from rivers and streams can significantly influence surface salinity. The freshwater carries its own salinity level, and when it mixes with the ocean, it can alter the overall salinity in these regions.
Polar Region Dynamics
Freezing and Thawing Influence on Surface Salinity:** In polar regions, the freezing and melting of ice play a pivotal role. As ice forms, it excludes salt, making the surrounding seawater saltier. Conversely, when the ice melts, it contributes freshwater, lowering salinity levels.
Wind-Driven Salinity Shifts
Wind can cause surface water to move, leading to the displacement of water masses. This movement can impact salinity by redistributing saltier or fresher water to different areas, thus altering local salinity levels.
Ocean Currents’ Contribution to Salinity Variability
Ocean currents transport water across vast distances. These currents can carry water with varying salinity levels, which can then mix with the local water, leading to changes in surface salinity.
Density and Temperature Changes
Changes in water density and temperature are closely related to salinity. When water becomes denser due to factors like cooling or increased salinity, it tends to sink. Conversely, when water becomes less dense due to warming or dilution with freshwater, it rises. These processes can lead to vertical mixing of water layers, affecting local salinity profiles.
Together, these factors demonstrate the intricate web of interactions between salinity, temperature, and density in ocean surface waters, highlighting the dynamic nature of our oceans and the importance of understanding these processes for various environmental and climatic studies.
READ MOREHeat Budget (Important Notes For APSC): Ocean salinity (Important for APSC)
| Highest salinity in water bodies | |
| Lake Van in Turkey | 330 o/oo |
| Dead Sea | 238 o/oo |
| Great Salt Lake | 220 o/oo |
| Dissolved Salts in Sea Water (gm of Salt per kg of Water) | |
| Chlorine | 18.97 |
| Sodium | 10.47 |
| Sulphate | 2.65 |
| Magnesium | 1.28 |
| Calcium | 0.41 |
| Potassium | 0.38 |
| Bicarbonate | 0.14 |
| Bromine | 0.06 |
| Borate | 0.02 |
| Strontium | 0.01 |
Role of ocean salinity:
Evaporation’s Impact on Surface Salinity
When water in the ocean’s surface layer undergoes evaporation, fresh water is removed, leaving behind the dissolved salts. This process increases the salinity of the remaining water.
Precipitation’s Role in Surface Salinity
Conversely, precipitation introduces fresh water into the ocean. Rainfall dilutes the salinity of the surface layer, as it adds relatively salt-free water to the mix.
Coastal Freshwater Inflow
In coastal areas, the flow of freshwater from rivers and streams can significantly influence surface salinity. The freshwater carries its own salinity level, and when it mixes with the ocean, it can alter the overall salinity in these regions.
Freezing and Thawing Influence on Surface Salinity
In polar regions, the freezing and melting of ice play a pivotal role. As ice forms, it excludes salt, making the surrounding seawater saltier. Conversely, when the ice melts, it contributes freshwater, lowering salinity levels.
Wind-Driven Salinity Shifts
Wind can cause surface water to move, leading to the displacement of water masses. This movement can impact salinity by redistributing saltier or fresher water to different areas, thus altering local salinity levels.
Ocean Currents’ Contribution to Salinity Variability
Ocean currents transport water across vast distances. These currents can carry water with varying salinity levels, which can then mix with the local water, leading to changes in surface salinity.
Density and Temperature Changes
Changes in water density and temperature are closely related to salinity. When water becomes denser due to factors like cooling or increased salinity, it tends to sink. Conversely, when water becomes less dense due to warming or dilution with freshwater, it rises. These processes can lead to vertical mixing of water layers, affecting local salinity profiles.
Together, these factors demonstrate the intricate web of interactions between salinity, temperature, and density in ocean surface waters, highlighting the dynamic nature of our oceans and the importance of understanding these processes for various environmental and climatic studies.
| Share of different salts |
| Sodium chloride — 77.7% |
| Magnesium chloride—10.9% |
| Magnesium sulphate —.4.7% |
| Calcium sulphate — 3.6% |
| Potassium sulphate — 2.5% |
Measuring Salinity
Salinity is a measure of the salt content in seawater and is typically expressed as parts per thousand (ppt). To calculate salinity, you determine the amount of salt dissolved in 1 kilogram (kg) of seawater. This measurement is essential for understanding the composition of seawater and its various properties.
Properties of Saltwater
The presence of dissolved salt in seawater has several significant impacts:
- Density: Saltwater is denser than freshwater due to the added solute (salt). This density difference affects ocean circulation and the distribution of marine life.
- Freezing Point: Saltwater freezes at lower temperatures compared to freshwater. This property is why oceans and seas with higher salinity levels take longer to freeze, making them more resistant to freezing in colder climates.
Regions with High Salinity
High salinity in oceans and seas is often associated with specific environmental factors:
- Atlantic Ocean: The Atlantic Ocean typically experiences high ocean salinity. This is because it receives relatively little rainfall, and strong winds contribute to increased evaporation, leaving behind more salt in the water.
- Mediterranean Sea: The Mediterranean Sea is known for its high salinity levels, often exceeding 38 ppt. This is attributed to its proximity to the open sea, high evaporation rates, limited rainfall, and minimal freshwater input. It acts as a natural “salt trap.”
Maintaining ocean salinity in a range of 34 to 36 ppt is crucial for the overall balance of marine ecosystems. This balance is achieved through processes like evaporation and the addition of freshwater from rainfall and rivers.
Regions with Low Salinity
Low salinity in ocean water is usually influenced by the influx of freshwater from various sources:
- Coastal Waters: Coastal areas typically have lower salinity near the land surface because freshwater from rivers, streams, and groundwater discharge into the ocean. This influx dilutes the seawater with less saline freshwater.
- Antarctic Ocean: He ocean surrounding Antarctica maintains relatively low salinity levels, typically below 34 ppt. This is because the cold temperatures and ice formation in the region reduce the salt concentration.
- Arctic Ocean: Similar to the Antarctic Ocean, the Arctic Ocean exhibits lower salinity, often below 30 ppt. This is due to the influence of melting ice and freshwater input from rivers. Icebergs, formed from land-based ice sheets with minimal salt content, contribute freshwater to the sea. When seawater freezes, the ice that forms contains very little salt, effectively removing salt from the ocean.
- Baltic Sea: The Baltic Sea, located in northern Europe and Scandinavia, has notably low salinity levels, frequently measuring less than 10 ppt. This low salinity is primarily due to the significant inflow of freshwater from hundreds of rivers that drain into the sea, diluting the salt content.
Horizontal Distribution of Salinity
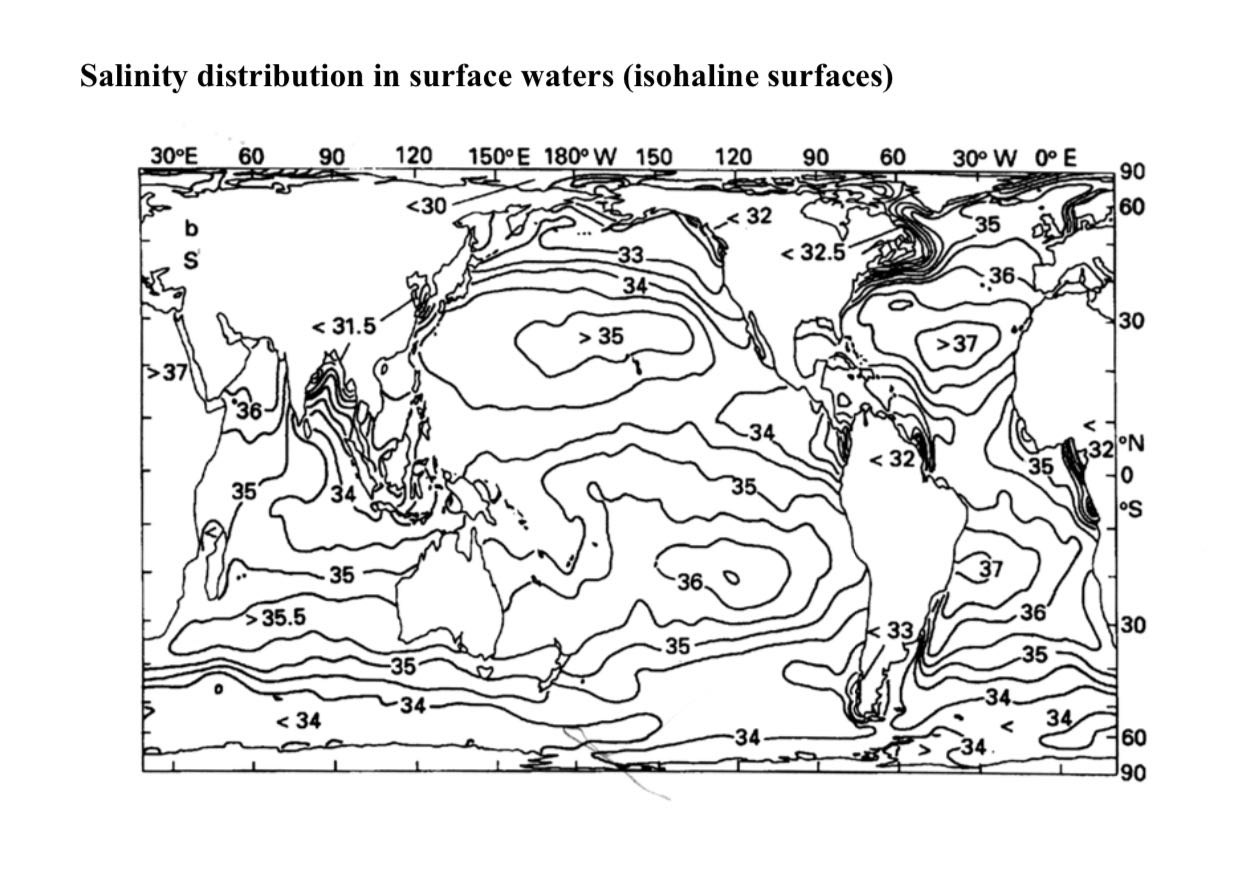
Normal Open Ocean Salinity (33-37)
- In the open ocean, the typical range of salinity falls between 33 and 37 parts per thousand (ppt).
Pacific Ocean
- The Pacific Ocean’s shape and vast size contribute to its varied salinity patterns. The distribution of landmasses and ocean currents across this extensive expanse results in differing salinity levels.
Atlantic Ocean
- The Atlantic Ocean generally maintains an average salinity level of around 36-37 ppt.
- Near the equator in the Atlantic Ocean, the salinity is approximately 35 ppt. This is because of heavy rainfall, high relative humidity, cloudiness, and the calm air of the doldrums, all of which reduce evaporation and contribute freshwater to the region.
- The polar regions, both in the Arctic and Antarctic, exhibit lower salinity due to minimal evaporation and significant freshwater input from ice melting. Salinity in these areas typically ranges from 20 to 32 ppt.
- Between the latitudes of 20°N and 30°N and longitudes of 20°W to 60°W, the Atlantic Ocean reaches its maximum salinity of 37 ppt. Salinity gradually decreases as you move northward from this region.
Indian Ocean
- The Indian Ocean generally maintains an average salinity level of 35 ppt.
- The Bay of Bengal, positioned to the northeast of the Indian Ocean, experiences lower salinity levels. This is primarily due to the substantial influx of freshwater from the Ganges River and other regional rivers.
- Conversely, the Arabian Sea, located to the northwest of the Indian Ocean, exhibits higher salinity levels. This is attributed to elevated evaporation rates and limited freshwater input.
Marginal Seas
- The North Sea, despite its northern location, records higher salinity levels due to the influence of more saline water brought by the North Atlantic Drift, a warm ocean current.
- The Baltic Sea, situated in northern Europe and Scandinavia, maintains low salinity levels. This is a consequence of the significant inflow of freshwater from numerous rivers that drain into the sea.
- The Mediterranean Sea exhibits high salinity levels primarily because of pronounced evaporation in the warm Mediterranean climate.
- The Black Sea records very low salinity due to substantial freshwater influx from rivers, particularly the Danube and Dniester.
Inland Seas and Lakes
- Inland seas and lakes, such as the Great Salt Lake in Utah, the Dead Sea, and Lake Van in Turkey, have extremely high salinity levels. This is due to continuous salt input from rivers and the progressive concentration of salt through evaporation. These bodies of water have become saltier over time, with salinity levels exceeding that of normal seawater.
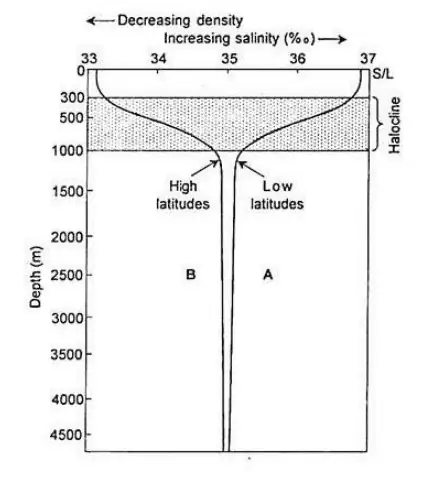
Vertical Distribution of Salinity
- Salinity in the ocean varies with depth, and this variation is influenced by factors such as latitude and the presence of cold and warm ocean currents.
- In high latitudes, salinity generally increases with depth, whereas in middle latitudes, it increases up to a certain depth (typically around 35 meters) before gradually decreasing. Near the equator, surface salinity is lower.
- Salinity at depth remains relatively constant as there is no significant addition or loss of water. However, there is a notable difference in salinity between the surface and deep ocean layers.
- A distinct zone known as the halocline, similar to the thermocline (temperature gradient), exhibits a sharp increase in salinity with depth.
- Increasing seawater salinity results in higher water density. Consequently, denser, high-salinity seawater tends to sink below less dense, lower-salinity water. This leads to stratification of seawater based on salinity, with distinct layers formed at different depths.
In our study of ocean salinity, we’ve explored both its vertical and horizontal distribution. Salinity, a measure of salt content, is typically calculated as the amount of salt dissolved in one kilogram (1 kg) or 1000 grams of seawater. These variations in salinity across different oceans are primarily influenced by their geographical locations and associated characteristics.
1. What is ocean salinity?
Ocean salinity refers to the concentration of dissolved salts, primarily sodium chloride (table salt), in seawater. It is typically expressed in parts per thousand (ppt).
2. How is ocean salinity measured?
Ocean salinity is measured using instruments like conductivity-temperature-depth (CTD) profilers, which detect the electrical conductivity of seawater to estimate its salinity. Other methods include chemical titration and the use of salinometers.
3. What is the average salinity of the world’s oceans?
The average salinity of the world’s oceans is approximately 35 parts per thousand (ppt).
4. Why does ocean salinity vary in different regions?
Ocean salinity varies due to factors such as precipitation, evaporation, freshwater inflow from rivers, melting ice, ocean currents, and geographical location. Different regions experience these factors to varying degrees.
5. Which ocean has the highest salinity?
The Red Sea, located between northeastern Africa and the Arabian Peninsula, has one of the highest ocean salinity levels, averaging around 41 ppt.